For Appointments & Consultations Call Us Today:
Spatial Thermal Imaging (STI)
Spatial Thermal Imaging Proof of Concept
ThermEval.com (sti@thermeval.com)
SUMMARY
Spatial Thermal Imaging© (STI) is a non-invasive imaging technique for visualizing internal anatomical details of the breast, with resolution comparable to MRI, This study appraised STI functionality and efficacy for revealing early tumors, and for differentiating between malignant and benign lesions. Studies of 1,675 clinically asymptomatic patients revealed 222 suspicious possible malignant neoplasms. Probable malignancies discovered with STI were confirmed by MRI yielding a 95.3% Positive Predictive Value.
STI is an essential adjuvant to traditional breast thermography, as it reveals the underlying causes of previously unexplained thermal signs associated with early breast cancer.
STI visualizes malignancies years before they are detectable with mammography, and evidences the functional capability for detecting early signs of possible nascent malignancies in the preneoplastic stages of tumor development, by the third year of tumor life.
STI possesses potential to compete with mammography as a cost-effective primary breast cancer screening modality.
MOTIVATION
Breast cancer is the second leading cause of cancer death among women. It is estimated more than 250,000 United States women will be diagnosed with the disease, and more than 40,000 will die of it each year 1.
Early detection of breast cancer lends itself to therapies which dramatically improve breast cancer survival. However, statistics gathered from over two decades demonstrate traditional screening hasn’t significantly altered breast cancer mortality rate 2-4.
Screening for early detection of breast cancer remains the best hope for reducing metastasis and improving mortality related to the disease. But the static mortality rate suggests current screening modalities are unsuccessful at detecting tumors early enough to affect appropriate therapeutic response. Improved detection and diagnostic techniques must be made available if a positive change can be expected.
Mammography is the standard for breast cancer screening, although its benefits and harms have been widely disputed in recent years 5,6. Known limitations and ineffectiveness of mammography 7 to detect tumors early enough to affect mortality and metastasis underscore the need for a new primary screening tool.
Thermal imaging, aka breast thermography is uniquely capable of showing early alterations of vascularity and cellular morphology, years before morphological changes are discoverable with mammography 9. Although severely abnormal thermal images are associated with a high probability of malignancy, sensitivity related to mildly abnormal thermal images suffers dramatically, as several benign conditions exhibit the same thermal signs as signs of early breast cancer.
STI was created with the intent of revealing the underlying causes of many early signs of breast disease, which are undetectable with mammography, and unexplained or dismissed with breast thermography alone.
STI virtually peels away tissue to reveal subcutaneous anatomical details from a standard thermal image. These visualizations disclose details that otherwise would remain hidden, and seeing those details brings clearer understanding of the breast condition. Simply put, seeing more means knowing more.
This Proof of Concept study was performed to demonstrate STI efficacy consistent with its objectives, and to evaluate STI potential as a viable replacement for current primary screening modalities.
THERMAL IMAGING
Breast Thermography, the ‘traditional’ thermal examination, calculates risk (probability) of developed malignancy based on the presence of twenty possible quantitative thermal signs statistically known to be associated with breast cancer. This objective methodology comports with Bayes Theorem, i.e., the more signs observed in the recorded images, the higher the probability of developed pathology. This objective analysis has significantly lower error rates than subjective image.
Assessed risk is categorized with the Marseille System of classification (TH1 – TH5), where each successive category represents an increased risk for developed breast cancer:
Specious claims abound that traditional breast thermography detects cancer years before mammography. Indeed, breast thermography reveals thermal signs statistically associated with early breast cancer, and a high-risk finding is associated with a high probability of malignancy. But, low-risk findings document only a small number of thermal signs related to breast cancer, and are thus ambiguous, as these same signs are also associated with myriad benign causes, e.g., a healthy breast, cysts, in situ carcinomas, etc.
METHODOLOGY
Traditional breast thermography is based on observations of the macro-characteristics of surface thermal patterns to compute the probability, i.e., risk of developed disease, whereas STI processes thousands of individual image pixels to visualize anatomical features of the subcutaneous breast.
STI treats thermal patterns as a set of thermal virtual wave fronts 8, each directly related to heat flow from individual, internal thermal sources. The temperature of each recorded pixel is the solution of the hyperbolic heat equation for a one dimensional longitudinal heat flow of a virtual wave emanating from internal thermal sources 9. This perception offers an opportunity for visualizing anatomical properties of subcutaneous heat sources by reverse modeling heat flow measured at the breast surface.
Solution of the heat equation is possible only when detailed knowledge of initial values, thermodynamic properties of the breast, its structure, properties of its microvascular network, heat source depth, and dimensions of the subject internal breast tissue are available. The proprietary STI algorithm overcomes these obstacles, processes each image pixel, performs reconstruction, and presents an image for viewing.
The Proof of Concept study was performed by Thermogram Assessment Services (TAS), the ThermEval affiliated image interpretation service. Images were sourced from TAS clients’ examination submissions, offering the benefits of a random population of symptomatic and asymptomatic patients, and images captured at low to high resolution.
1,675 thermal images were evaluated. Each was first assessed for risk of developed malignancy using traditional breast thermography, then each image was studied with STI to detect tissue suspected of possible malignancy. The probability of significant pathology was classified consistent with characteristics of the revealed suspicious tissue.
Unique characteristics of tumors and their anatomical and functional effects enables objective assessment of their pathological importance, permitting its categorization from ‘very unlikely serious’ to ‘likely serious’ pathology.’
Classification of suspicious tissue visualized with STI was dependent on multiple factors, such as a first-degree association with a vessel, quantitative measurements, specific image macro-characteristics, risk assessment, and morphological characteristics.
The primary features of malignant tissue, weighted corresponding to a feature’s likeliness of pathological implication are, in ascending order of pathological importance:
- First-degree association with a blood vessel: An association with a blood source is essential for tumor nourishment, a suspicious mass without a vascular association disqualified it as a possible malignancy.
- Focused Vascularity: Multiple vessels focused in the region of a malignancy indicate increased blood flow to that region. (Focused vascularity may ambiguously appear with benign lesions, e.g., cysts, and thus have reduced impact on identification as a malignancy.)
- Hyperthermia: Angiogenesis, vasculogenesis, increased vascularity, and metabolic processing elevate the local, and by extension the surrounding region of a malignant tumor. Suspicious tissue elevated ≥ 1ºC more than he contiguous region is considered hyperthermia.
- Irregular geometric structure, with a jagged exterior border: Malignant tumor cells randomly pierce the tumor’s basal lamina boundary, resulting n an irregular tumor shape with jagged edges. Non-malignancies are generally geometrically regular, with smooth surfaces.
- Abnormal TH4 or TH5 risk assessment: Statically-derived high risk assessment is an unequivocal prognosticator for development of breast cancer, indicating serious pathology exists within the breast.
- Tortuous vessel structure: Arterial and venous plexuses associated with more advanced malignancies present as extremely anarchic structures, evidencing angiogenesis.
- Distorted local vessel structure: Young tumors precede development of significant plexuses; however, nitrous oxide exuded from aggressive cancer into the blood stream alters micro-circulation distorting vessel structure. Vessels become dilated and elongated10, rendering vessels associated with the tumor anfractuous 11.
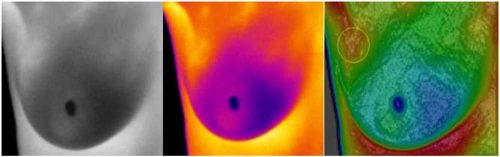
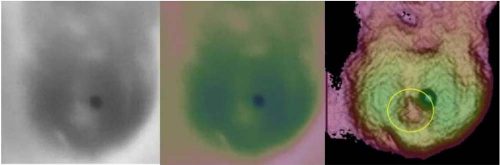
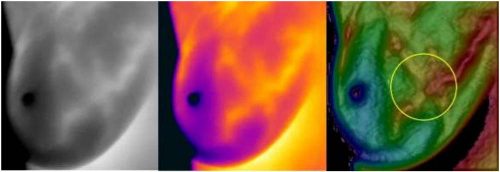
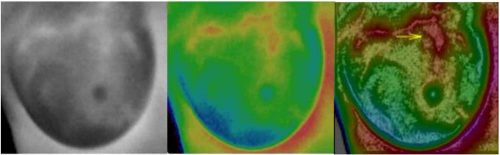
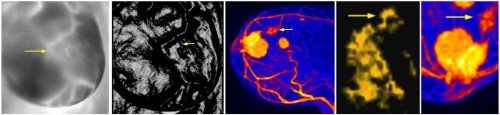
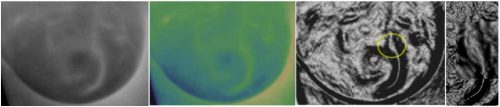
Appendix 1 shows several sample images.
The equation employed to calculate the ‘Mass Score’ is the sum of the weighted scores of each observed parameter.
Mass Score is divided into six categories, M1 – M6, classifying the probability of malignancy for suspicious tissue as follows:
RESULTS
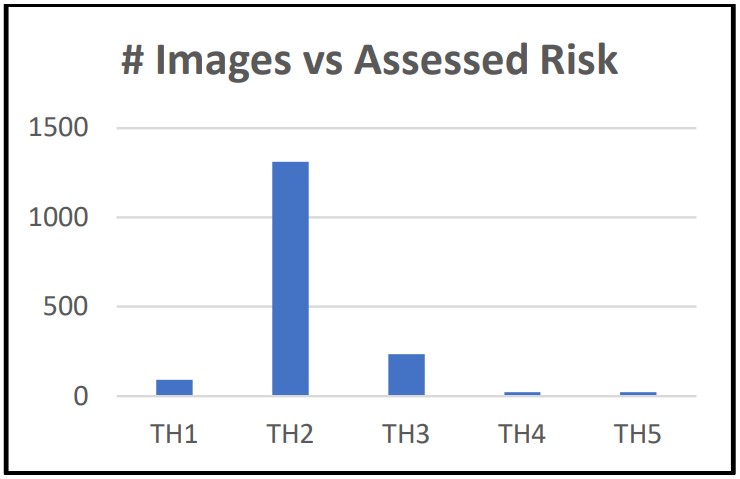
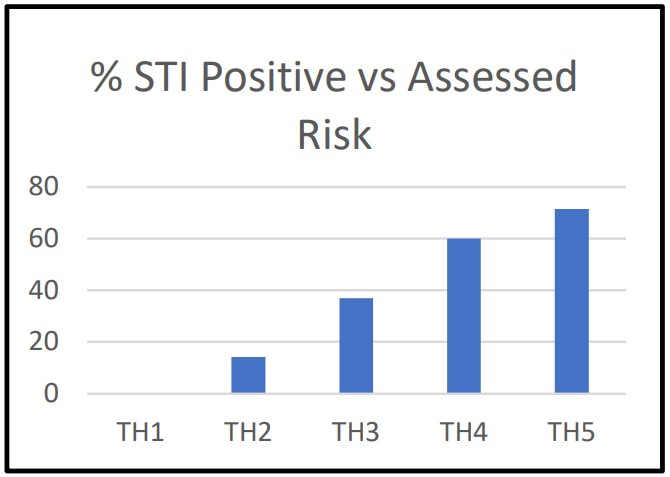
Of 1,675 images evaluated, 298 were revealed by STI as suspicious tissue. 76 were classified unlikely malignant, and 222 indicated possible malignancy (M3 – M5).
STI results were compared to breast thermography risk. The following table and charts summarize the study results.

CONCLUSIONS
Conclusions from this Proof of Concept study demonstrates STI:
- Efficacy in identifying early-stage malignancies
- Capability for revealing small, malignant tumors, less than 2mm diameter
- Capacity for detecting malignancies years before current primary screening modalities
- Capability for resolving ambiguities in low risk assessments by revealing the underlying causes of observed thermal signs
- Is a valuable and essential adjuvant to traditional breast thermography
- Uncovered a high incidence of malignancies in low risk patients. Approximately 10% of patients with TH2 assessments and 26% of patients with TH3 assessments were found to be harboring malignancies
- Revealed 222 tumors identified as possibly malignant were confirmed in patients undergoing a MRI follow-up, yielding a 95.3% Positive Predictive Value
- Potential as a suitable replacement for mammography as the primary breast cancer screening modality.
APPENDIX 1. Sample Images
The following are examples of STI visualizing subcutaneous lesions. Shown in order from left to right are the raw 255-level grayscale image, same raw image false colored, and the STI image.
APPENDIX 2. Work-in-Process
STI for Earliest Malignancy Detection
STI exceeded expectations by revealing signs of malignancy years before visualization of developed tumors.
The median size of breast tumors detected with high-quality mammography is 1.0 cm – 1.5 cm diameter 12, about 7 years after inception 1. The average size of MRI- and STI 2 – visualized malignant tumors is 2 mm 13, about five years after inception.
Significant benefits are derived by earlier visualization of mature malignant tumors. Identification of nascent malignancies offers even greater benefits. The detection of early tumors lessens the chance of metastasis, abbreviates surgical intervention, and dramatically improves mortality. STI is a method for detecting nascent tumors, typically by the third year of tumor life.
Neovascularization 15, i.e., angiogenesis, vasculogenesis, vasculogenic mimicry 16 is essential to the development of a tumor17. In combination with lymphangiogenesis 18, there is increased possibility of malignant cells metastasizing. Neovascularization is activated during the early, preneoplastic stages in the development of a tumor 19, typically in the second year of tumor life.
Neovascularization increases tumoral perfusion, which in turn, increases metabolic heat generation 20. The heat propagating to the surface of the breast is visibly indiscernible; however, STI is capable of detecting the heat, allowing identification of the morphological signatures of angiogenesis and vasculogenesis.
When STI has revealed signs of suspected neovascularization, it can be confirmed by employing MRI with detectable contrast media to label endothelial progenitor cells 21. Confirmation of neovascularization gives rise to potential employment of non-systemic antiangiogenic therapeutic methods 22.
Clinical Trial
To confirm STI efficacy, 1,000 clinically asymptomatic patients will be examined with traditional thermography, STI, ultrasound, mammography, and MRI in an interventional clinical trial. STI 95% Positive Predictive Value will undergo independent verification, Negative Predictive Value, Sensitivity, and Specificity will be determined, and STI ability to detect the signs of neovascularization during the preneoplastic stages will be ascertained. Cost of the six-month clinical trial is estimated to be $1 million, funded by grant.
1 Mammography is capable of revealing 1mm microcalcifications; however, they are inferential and inconclusive 14.
2 Appendix 1, Figure 4 – Figure 6, show STI tumor visualization is comparable to MRI.
References:
1. Breast Cancer Statistics, www.breastcancer.org/symptoms/understand_bc/statistics.
2. Breast Cancer Screening, Incidence, and Mortality Across US Counties Charles Harding et al, http://wilsonweb.physics.harvard.edu/publications/940ref.pdf.
3. Effect of Screening Mammography on Cancer Incidence and Mortality JAMA Intern Med. 2015;175(9):1490-1491, http://archinte.jamanetwork.com/article.aspx?articleid=2363022.
4. Annual Screening Does Not Reduce Risk of Death From Breast Cancer, http://www.medicalnewstoday.com/articles/272433.php.
5. Benefits and Harms of Mammography Screening, Breast Cancer Res. 2015; 17(1): 63, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4415291/.
6. National Cancer Institute Fact Sheet – “Mammograms”, https://www.cancer.gov/types/breast/mammograms-fact-sheet (What are the benefits and potential harms of screening mammograms?).
7. Chris Kresser, The Downside of Mammograms, https://kresserinstitute.com/thedownside-of-mammograms/, April 6, 2017.
8. P. Burgholzer et al; Three-dimensional Thermographic Imaging Using a Virtual Wave Concept, https://arxiv.org/ftp/arxiv/papers/1610/1610.00868.pdf.
9. R.K.Sahoo; Propagation of Thermal Waves with Lateral Heat Transfer, https://doi.org/10.1016/0011-2275(94)90170-8.
10. Konerding MA, Malkusch W, Klapthor B, van Ackern C, Fait E, Hill SA, Parkins C, Chaplin DJ, Presta M, Denekamp J. Evidence for characteristic vascular patterns in solid tumors: Quantitative studies using corrosion casts. British Journal of Cancer. 1999;80(5-6):724.
11. Li C‐Y, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial Stages of Tumor Cell‐induced Angiogenesis: Evaluation via Skin Window Chambers in Rodent Models. Journal of the National Cancer Institute. 2000;92(2):143-147, https://doi.org/10.1093/jnci/92.2.143.
12. H. Gilbert Welch et al, Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. NEJM 2016; 375:1438-1447, https://www.nejm.org/doi/full/10.1056/NEJMoa1600249?utm_medium=referral&utm_source=r360.
13. Anne-julie Carin et al, Relevance of breast MRI in determining the size and focality of invasive breast cancer treated by mastectomy: a prospective study, World J Surg Oncol. 2017; 15: 128, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513043/.
14. P.Henro et al, Breast microcalcifications: The lesions in anatomical pathology,
Diagnostic and Interventional Imaging Volume 95, Issue 2, February 2014, Pages 141-152.
15. Naoto Nishida et al, Angiogenesis in Cancer, Vascular Health Risk Management, 2006 Sep; 2(3): 213–219, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1993983/.
16. Luo, Q., Wang, J., Zhao, W. et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol 13, 19 (2020), https://doi.org/10.1186/s13045-020-00858-6.
17. Iratxe Zuazo-Gaztelu & Oriol Casanovas, Unraveling the Role of Angiogenesis inCancer Ecosystems, https://www.frontiersin.org/articles/10.3389/fonc.2018.00248/full.
18. Munazzah Rahman & Sulma Mohammed, Breast cancer metastasis and the lymphatic system, Oncol Lett. 2015 Sep; 10(3): 1233–1239.
19. D Hanahan & J Folkman, Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis, Cell. 1996 Aug 9;86(3):353-64, https://doi.org/10.1016/S0092-8674(00)80108-7.
20. Lozano, A. et al. Determining the thermal characteristics of breast cancer based on high-resolution infrared imaging, 3D breast scans, and magnetic resonance imaging. Sci Rep 10, 10105 (2020), https://doi.org/10.1038/s41598-020-66926-6.
21. Agudelo CA, Yamaoka T. Magnetic resonance imaging detection of an uncommon granuloma formation after endothelial progenitor cells transplantation. Biomed Mater Eng. 2013;23(6):555-66, https://doi.org/10.3233/BME-130768.
22. Fakhrejahani, E., & Toi, M. (2014). Antiangiogenesis therapy for breast cancer: an update and perspectives from clinical trials. Japanese journal of clinical oncology, 44(3), 197–207, https://doi.org/10.1093/jjco/hyt201.